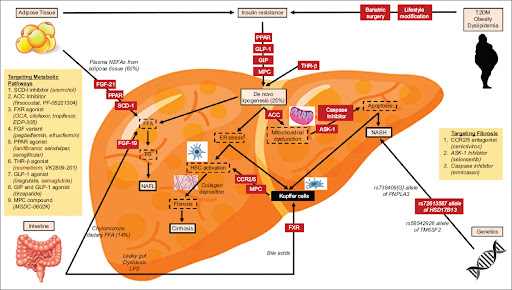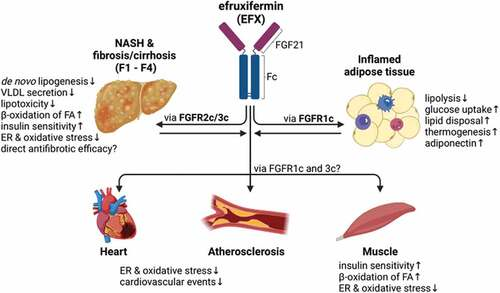
The New England Journal of Medicine recently published a study titled “Efruxifermin in Compensated Liver Cirrhosis Caused by MASH” on May 7, 2025. This phase 3 clinical trial evaluated the efficacy and safety of efruxifermin, a fibroblast growth factor 21 (FGF21) analog, in patients with compensated liver cirrhosis due to metabolic dysfunction–associated steatohepatitis (MASH).New England Journal of Medicine+3New England Journal of Medicine+3New England Journal of Medicine+3New England Journal of Medicine
Study Design and Participants:
The trial enrolled 800 patients diagnosed with compensated liver cirrhosis resulting from MASH. Participants were randomized to receive once-weekly subcutaneous injections of efruxifermin or placebo over a 36-week period.New England Journal of Medicine
Primary and Secondary Outcomes:
- Primary Outcome: The main objective was to assess the proportion of patients achieving at least a one-stage improvement in liver fibrosis without worsening of MASH by week 36.
- Secondary Outcomes: These included additional histological improvements, changes in liver-related biomarkers, and the incidence of clinical events such as hepatic decompensation, liver transplantation or qualification for transplantation, and all-cause mortality.New England Journal of MedicineNew England Journal of Medicine+1New England Journal of Medicine+1
Key Findings:
The study found that a higher percentage of patients treated with efruxifermin experienced significant improvements in liver fibrosis without progression of MASH compared to the placebo group. Additionally, the efruxifermin group showed favorable changes in liver biomarkers and a lower incidence of clinical deterioration events.New England Journal of Medicine+3New England Journal of Medicine+3New England Journal of Medicine+3
Safety Profile:
Efruxifermin was generally well-tolerated. The most common adverse events reported were mild to moderate gastrointestinal symptoms, with no significant differences in serious adverse events between the treatment and placebo groups.
Conclusion:
Efruxifermin demonstrates potential as an effective treatment for patients with compensated liver cirrhosis due to MASH, offering improvements in liver fibrosis and a favorable safety profile. These findings support further investigation into its long-term benefits and potential role in clinical practice.